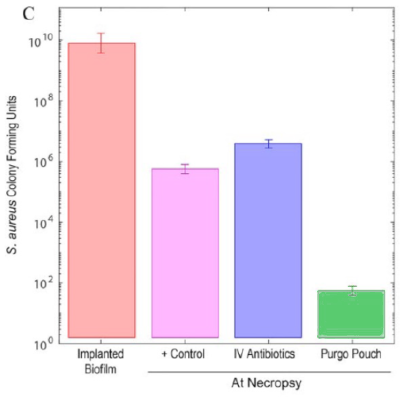
Demonstrated Efficacy – Pre-Clinical with Study Sustained Delivery Device
Demonstrated Efficacy – Pre-Clinical Study with
Sustained Delivery Device
9 Sheep Implanted With a Biofilm-contaminated Plate
(each with ~3 billion bacteria)
9 Sheep Implanted
With a Biofilm-contaminated Plate
(each with
~3 billion bacteria)
TREATMENT
RESULTS AFTER 21 DAYS
2 sheep received NO ANTIBIOTICS as control
More than 100,000,000 bacteria
INFECTED
2 sheep received
NO ANTIBIOTICS
as control
More than 100,000,000 bacteria
INFECTED
2 sheep received ANTIBIOTICS INTRAVENOUSLY
More than 100,000,000 bacteria
INFECTED
2 sheep received
ANTIBIOTICS
INTRAVENOUSLY
More than 100,000,000 bacteria
INFECTED
5 sheep received ANTIBIOTICS VIA PURGO POUCH
Less than 1,000 bacteria
NOT INFECTED*
5 sheep received
ANTIBIOTICS VIA
PURGO POUCH
Less than 1,000 bacteria
NOT INFECTED*
* Infectious dose is clinically defined as 105 CFU or 100,000 bacteria.
TREATMENT
2 sheep received
NO ANTIBIOTICS as control
RESULTS AFTER 21 DAYS
More than 100,000,000 bacteria
INFECTED
TREATMENT
2 sheep received
ANTIBIOTICS INTRAVENOUSLY
RESULTS AFTER 21 DAYS
More than 100,000,000 bacteria
INFECTED
TREATMENT
5 sheep received
ANTIBIOTICS VIA PURGO POUCH
RESULTS AFTER 21 DAYS
Less than 1,000 bacteria
NOT INFECTED*
* Infectious dose is clinically defined as
105 CFU or 100,000 bacteria.
Superior to Existing Options
Superior to
Existing Options
| Surgical Site Infection Treatment Options | Purgo Pouch Sustained Delivery Device | Oral or IV Antibiotics Administration | Localized Antibiotic Infusion | Stimulan Beads with Antibiotics | Antibiotic Powders Placed in Wound or Surgical Incision |
| Effective to Control Planktonic Bacteria |
|
|
|
|
|
| Adjustable Dosing |
|
|
|
X | X |
| Flexibility to Change Antibiotics |
|
|
|
X | X |
| Daily at Home Management |
|
|
X | X | X |
| *Effective Biofilm Eradication |
|
X | X | X | X |
*As demonstrated in sheep models.